electronic configuration of bromine 35|Complete Electron Configuration for Bromine (Br, Br : Baguio Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa Zeus138 menawarkan berbagai game slots, togel, poker, sportsbook, tangkas dan live casino. ☰ LOGIN DAFTAR Zeus138 | Link Zeus 138 Slot Login | Daftar Zeus138 Asia. LOGIN DAFTAR. DETAIL AGEN . Nama Agen: Zeus138: Bahasa: Indonesia, English: Mata Uang: IDR (Rupiah Indonesia) Deposit Minimal: Rp 10,000 (Sepuluh Ribu Rupiah) .
PH0 · Electronic configuration of bromine ?
PH1 · Electron configuration for Bromine (element 35). Orbital diagram
PH2 · Electron Configuration Chart of All Elements (Full Chart)
PH3 · Complete Electron Configuration for Bromine (Br, Br
PH4 · Bromine electron configuration
PH5 · Bromine Electron Configuration (Br) with Orbital Diagram
PH6 · Bromine (Br) [35] — Chemical Element — Periodic Table
PH7 · Bromine
PH8 · Atomic Number 35
In most EuroMillions countries, the Millionaire Maker prize is €1 million. Swiss players receive an amount of equivalent value in Swiss Francs (CHF), based on the exchange rate at the time. For example, on 23rd February 2018, a .
electronic configuration of bromine 35*******Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of bromine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. In the bromine ground-state electron configuration, the last five electrons of the 4p orbital are located in the . Tingnan ang higit paelectronic configuration of bromine 35The total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paThe electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of bromineare seven. The elements that have 5, 6, or 7 electrons in the last . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Mar 23, 2023 Melting point: -7.3 ℃. Density: 3.14 g/cm 3 . Electronic configuration of the Bromine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p .Allotropes. Br. Bromine. 35. 79.904. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations . Bromine Electron Configuration: Bromine (Br) is a chemical element. The atomic number of bromine is 35. It is the fuming red-brown liquid at room temperature and the third-lightest halogen. It .
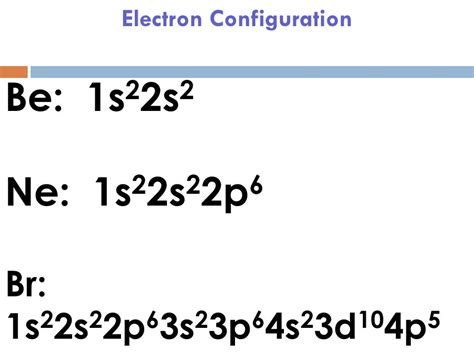
35: Group: group 17 (halogens) Period: period 4: Block p-block: Electron configuration 3d 10 4s 2 4p 5: Electrons per shell: 2, 8, 18, 7: Physical properties; Phase at STP: liquid: Melting point (Br 2) 265.8 K (−7.2 °C, . The electron configuration of Bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [Ar]4s23d104p5. Explanation: Use a chart such as the one below to fill the subshells in .
Physical data. Electronic data. Shells: 2, 8, 18, 7. Orbitals: [Ar] 3d 4s 4p. Electronegativity: 2.8, 2.7. 1. Ionization potential: 11.8138 eV. 2. Ionization potential: 21.8 eV. 3. Ionization .
Atomic Number: 35. Name: Bromine. Atomic symbol: Br. Atomic Weight: 79.904. Electron affinity: -325 kJ/mol. Electron configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. .
Since the atomic number of bromine is 35, the total electrons of bromine are 35. Second, make a table of subshell and its maximum electrons. Calculate the maximum number of electrons each .
The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be . But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p. Oganesson (element 118) is a good example to show .Get the facts about element Bromine (Br) [35] from the periodic table. . electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. Periodic Table of the Elements; Bromine: Non-Metal: Symbol: Br Atomic number: 35 Atomic mass: 79.904 Group: Halogen CAS-number .Element 35 of Periodic table is Bromine with atomic number 35, atomic weight 79.904. Bromine, symbol Br, has a Base Centered Orthorhombic structure and Red color. Bromine is a Halogens element. It is part of group 17 (fluorine family). Know everything about Bromine Facts, Physical Properties, Chemical Properties, Electronic configuration .electronic configuration of bromine 35 Complete Electron Configuration for Bromine (Br, BrUse an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms: N, Si, Fe, Te, and Mo; Write the full orbital diagram for fluorine. Write the orbital diagram for Cd2+. Write the orbital diagram for Cd2+. Write the electron configuration for bromine and state the number of valence electrons.

La configuración electrónica del bromo es: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. El bromo, también conocido como fuego líquido, se define como un elemento químico que forma parte de la tabla periódica de elementos. Su número atómico es 35, se ubica en el grupo de los halógenos, más precisamente en el grupo VII A. Br es su .Complete Electron Configuration for Bromine (Br, BrLa configuración electrónica del bromo es: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. El bromo, también conocido como fuego líquido, se define como un elemento químico que forma parte de la tabla periódica de elementos. Su número atómico es 35, se ubica en el grupo de los halógenos, más precisamente en el grupo VII A. Br es su .
There are 35 arrows in the Electron Configuration For Bromine, which is for orbital filling. These 35 arrows of bromine are only due to the atomic number of bromine. The orbital filling order is 1s, 2s, 3p, 3s, 4s, 3d, 4p, etc. You can see that 3p is coming before the 4p and after the 4s. We will fill the lowest energy level first, and it will .Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Using the blocks in the periodic table we can write the electron configuration of bromine as: 1s22s22p63s23p64s23d104p5. If we were writing the electron configuration for the bromine anion, we would begin writing the same .Well, for bromine, Z = 35 and we use the Aufbau principle. Electronic configuration : 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 1 0 4 p 5 or [ A r ] 4 s 2 3 d 1 0 4 p 5 Electron configuration of Bromine (Br-35) - 9209178. Font 12 Paragraph 1 Se Replace No Spac. Heading 1 Heading 2 Dictate Sens -Select- E Styles Editing Voice Sens 2 3 4 6 Research on the formulas for c .The Electron configuration of bromine is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Bromine, also known as liquid fire, is defined as a chemical element that is part of the periodic table of elements. Its atomic number is 35, it is located in the group of halogens, more precisely in group VII A. Br is its atomic symbol. #1 Find electrons of bromine #2 Write electron configuration of bromine #3 Draw orbital diagram of bromine. Let’s break down each step in detail. . Since the atomic number of bromine is 35, the total electrons of bromine are 35. Write electron configuration. The electron configuration of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Bromine is 35. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative .Configuration électronique du brome. La configuration électronique d'un élément chimique est la représentation des électrons situés dans les sous-niveaux d'énergie, c'est-à-dire dans leur dernière orbite ou coquille. Le numéro atomique du brome est égal à 35. Pour cette raison, sa configuration électronique est : 1s² 2s² 2p⁶ .Electronic configuration of bromine is 1s22s22p63s23p64s23d104p5 Bromine Have 35 electrons. The 1s subshell in the first shell can hold up to two electrons at a time, which are positioned in the 1s.
The melting point of bromine is -7.2 °C and its boiling point is 58.8 °C. The atomic mass of bromine is 79.904 u and its density is 3.12 g/cm 3. Bromine has a very less number of isotopes. And out of them, only 2 isotopes are stable ( 79 Br and 81 Br). 79 Br has an abundance of 51% and 81 Br has an abundance of 49%.
Themed slots are more than just a game; they’re an experience, a journey into worlds we love, and an opportunity to win real money while enjoying our favorite stories and songs. They are the perfect blend of entertainment, nostalgia, and the thrill of casino gaming, wrapping players in a familiar yet exhilarating adventure with every spin.
electronic configuration of bromine 35|Complete Electron Configuration for Bromine (Br, Br